Overview
Designed to Deliver Excellence
Founded in 2015 in California, NeuroVasc Technologies brings over 100 years of combined expertise in neurovascular device design and development. We are committed to being the most efficient and innovative developer of neurovascular treatment devices in the world.
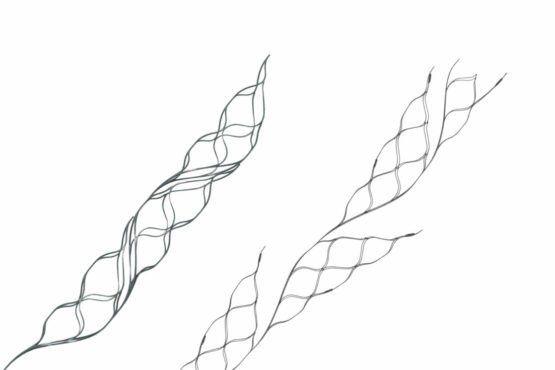
NeuroVasc is focused on developing a portfolio of novel catheter-based technologies to facilitate broad treatment options for patients suffering stroke and other neurovascular diseases.